| |
 Servometer– PMG, LLC introduced a new and unique “atherm” temperature sensitive bellows assembly. These lightweight, extremely sensitive assemblies are thin-walled, electrodeposited nickel bellows soldered to custom end pieces filled with a working fluid (with a known coefficient of thermal expansion) and then sealed. By sealing a specific volume of fluid within a Servometer electro-deposited nickel bellows, the volumetric thermal expansion characteristic of the fluid is transformed into a precise, measurable linear movement. Servometer– PMG, LLC introduced a new and unique “atherm” temperature sensitive bellows assembly. These lightweight, extremely sensitive assemblies are thin-walled, electrodeposited nickel bellows soldered to custom end pieces filled with a working fluid (with a known coefficient of thermal expansion) and then sealed. By sealing a specific volume of fluid within a Servometer electro-deposited nickel bellows, the volumetric thermal expansion characteristic of the fluid is transformed into a precise, measurable linear movement. |
|
 SEBRA introduces the Poco personal sealer, the next generation of ergonomic and eco-friendly sealers. This small, lightweight sealer is sized and priced to allow every phlebotomist to keep one on hand. Throw away your grommets and replace them with a more economical solution that provides the assurance of hermetic seals. The Poco sealer is for light duty use- it will produce 250 seals on blood bag tubing per battery charge. The environmentally safe NiMH battery is easy to use and is user replaceable. SEBRA introduces the Poco personal sealer, the next generation of ergonomic and eco-friendly sealers. This small, lightweight sealer is sized and priced to allow every phlebotomist to keep one on hand. Throw away your grommets and replace them with a more economical solution that provides the assurance of hermetic seals. The Poco sealer is for light duty use- it will produce 250 seals on blood bag tubing per battery charge. The environmentally safe NiMH battery is easy to use and is user replaceable. |
| • For more information please click here |
|
• For more information please click here |
| |
| |
 Machine Solutions Inc. (MSI) announced the North American launch of its new LB1000 Laser Bonding Equipment. The LB1000 offers advance process control and feedback via closed loop temperature and laser power control for proximal and distal balloon bonds as well as other catheter tube bonding applications. The ability to process based on bond site temperature simplifies profile development and minimizes product variability. Additional features include drop-in product loading, PLC controlled touch screen interface, optional automated bond site alignment, integrated vision assist system, cycle data acquisition and output with networking capability. Machine Solutions Inc. (MSI) announced the North American launch of its new LB1000 Laser Bonding Equipment. The LB1000 offers advance process control and feedback via closed loop temperature and laser power control for proximal and distal balloon bonds as well as other catheter tube bonding applications. The ability to process based on bond site temperature simplifies profile development and minimizes product variability. Additional features include drop-in product loading, PLC controlled touch screen interface, optional automated bond site alignment, integrated vision assist system, cycle data acquisition and output with networking capability. |
|
 Wacker Chemie AG and MorphoSys AG are intensifying their cooperation in the use of WACKER’s prokaryotic secretion technology. The two companies have signed a new agreement precisely defining areas of use and production limits under which MorphoSys will continue to use the secretion system on a research scale. As a result, MorphoSys will now be able to use the WACKER technology for both the early development phase of therapeutic projects as well as the production of diagnostic and research antibodies. Wacker Chemie AG and MorphoSys AG are intensifying their cooperation in the use of WACKER’s prokaryotic secretion technology. The two companies have signed a new agreement precisely defining areas of use and production limits under which MorphoSys will continue to use the secretion system on a research scale. As a result, MorphoSys will now be able to use the WACKER technology for both the early development phase of therapeutic projects as well as the production of diagnostic and research antibodies. |
| • For more information please click here |
|
• For more information please click here |
| |
| |
 The Lee VHS Injection Manifold Mount System allows for the precise injection of fluid into a flow stream. Using this system, any ported Lee VHS micro-dispense valve can be mounted so that the outlet port is in close proximity to the flow stream, thus minimizing captive capillary volume and increasing injected volume repeatability. Lee VHS valves are high-speed, two-way solenoid valves that have set the standard in applications requiring microliter and nanoliter dispense volumes. The Lee VHS Injection Manifold Mount System allows for the precise injection of fluid into a flow stream. Using this system, any ported Lee VHS micro-dispense valve can be mounted so that the outlet port is in close proximity to the flow stream, thus minimizing captive capillary volume and increasing injected volume repeatability. Lee VHS valves are high-speed, two-way solenoid valves that have set the standard in applications requiring microliter and nanoliter dispense volumes. |
|
 Henkel Corporation has introduced a line of single-component light and moisture curing silicone adhesives designed for use on medical devices that incorporate silicone materials or require highly flexible bond joints or coatings. All three products in the line cure within 30 seconds on exposure to a suitable UV/visible light source. These medical silicone adhesives eliminate part racking and allow immediate quality control testing. To ensure robust assemblies, the products offer high-bond strengths on silicone and plastics including polycarbonate. Henkel Corporation has introduced a line of single-component light and moisture curing silicone adhesives designed for use on medical devices that incorporate silicone materials or require highly flexible bond joints or coatings. All three products in the line cure within 30 seconds on exposure to a suitable UV/visible light source. These medical silicone adhesives eliminate part racking and allow immediate quality control testing. To ensure robust assemblies, the products offer high-bond strengths on silicone and plastics including polycarbonate. |
| • For more information please click here |
|
• For more information please click here |
| |
| |
 DEERFIELD, Ill— Baxter Healthcare Corporation announced that it received 510(k) clearance for expanded labeling for the first antimicrobial needleless intravenous (IV) connector, V-Link Luer -activated device (LAD) with VitalShield protective coating. With a new federal policy restricting reimbursement for healthcare-associated infections (HAIs) taking effect on October 1, healthcare professionals are seeking effective techniques and technologies to reduce the risk of contamination from a broad array of pathogens (infection-causing agents) within their hospitals. DEERFIELD, Ill— Baxter Healthcare Corporation announced that it received 510(k) clearance for expanded labeling for the first antimicrobial needleless intravenous (IV) connector, V-Link Luer -activated device (LAD) with VitalShield protective coating. With a new federal policy restricting reimbursement for healthcare-associated infections (HAIs) taking effect on October 1, healthcare professionals are seeking effective techniques and technologies to reduce the risk of contamination from a broad array of pathogens (infection-causing agents) within their hospitals. |
|
 The latest addition to Filtertek's industrial product line is their General Purpose In-Line Filters. Designed for use in a number of different applications, these products are available in various screen sizes and port configurations. The robust housing design withstands higher pressure situations and is resistant to alcohol and fuel. The color coded inside elements disclose the products filter rating for easy identification. Customization is available for different screen usage or port configurations in quantities over 20,000 pieces. The latest addition to Filtertek's industrial product line is their General Purpose In-Line Filters. Designed for use in a number of different applications, these products are available in various screen sizes and port configurations. The robust housing design withstands higher pressure situations and is resistant to alcohol and fuel. The color coded inside elements disclose the products filter rating for easy identification. Customization is available for different screen usage or port configurations in quantities over 20,000 pieces. |
| • For more information please click here |
|
• For more information please click here |
| |
| |
 St. Paul, MN- Biopharmaceutical process engineers seeking a secure and cost-effective aseptic disconnect from equipment now have a reliable, easy-to-use solution: HFC39 Series couplings from Colder Products Company. Extensively tested for biocompatibility and performance, the HFC39 Series couplings are designed to prevent external organisms from entering through the connector and into the flow path during disconnection. When integrated into pre-sterilized single use systems, the HFC39’s automatic shutoff valves allow an aseptic disconnection by closing off the flow path prior to fully disconnecting. St. Paul, MN- Biopharmaceutical process engineers seeking a secure and cost-effective aseptic disconnect from equipment now have a reliable, easy-to-use solution: HFC39 Series couplings from Colder Products Company. Extensively tested for biocompatibility and performance, the HFC39 Series couplings are designed to prevent external organisms from entering through the connector and into the flow path during disconnection. When integrated into pre-sterilized single use systems, the HFC39’s automatic shutoff valves allow an aseptic disconnection by closing off the flow path prior to fully disconnecting. |
|
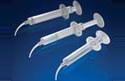 Edgewood, NY — Qosina announces the addition of a new dispensing syringe with a revolving curved tip This syringe is ideal for dispensing viscous materials into tight spaces requiring precise application. The one inch curved syringe tip is fitted onto the syringe body with a 360° rotation for maximum flexibility and directional adjustment. Our natural POM syringe has a three-milliliter draw and is chemically resistant to many solvents. For pre-filling, Qosina stocks a flexible syringe tip cap. Edgewood, NY — Qosina announces the addition of a new dispensing syringe with a revolving curved tip This syringe is ideal for dispensing viscous materials into tight spaces requiring precise application. The one inch curved syringe tip is fitted onto the syringe body with a 360° rotation for maximum flexibility and directional adjustment. Our natural POM syringe has a three-milliliter draw and is chemically resistant to many solvents. For pre-filling, Qosina stocks a flexible syringe tip cap. |
| • For more information please click here |
|
• For more information please click here |
| |
| |
 Carpinteria, California —NuSil Technology, a cutting-edge manufacturer of silicone-based materials, and Ciba, a worldwide provider of turnkey antimicrobial solutions to the medical device industry, announce their agreement to jointly provide antimicrobial solutions for use in medical device and healthcare-related silicone products. The goal of the collaboration between NuSil and Ciba is to develop customized medical silicone materials incorporated with an innovative microbial defense system that offers built-in broad range spectrum protection against growth of microorganisms — specific to any device application needs. Carpinteria, California —NuSil Technology, a cutting-edge manufacturer of silicone-based materials, and Ciba, a worldwide provider of turnkey antimicrobial solutions to the medical device industry, announce their agreement to jointly provide antimicrobial solutions for use in medical device and healthcare-related silicone products. The goal of the collaboration between NuSil and Ciba is to develop customized medical silicone materials incorporated with an innovative microbial defense system that offers built-in broad range spectrum protection against growth of microorganisms — specific to any device application needs. |
|
 Salt Lake City, Utah –ZEVEX Inc. has expanded its line of innovative enteral feeding products by launching the Infinity® Orange™ small volume enteral feeding pump. The Infinity Orange is an enteral-only feeding system that has been optimized for use in clinical intensive care units, homecare environments, or anywhere small volume feedings are administered. The Infinity Orange improves patient safety by effectively reducing the possibility of accidental feeding into an IV line or injection of IV medication into a feeding tube. Salt Lake City, Utah –ZEVEX Inc. has expanded its line of innovative enteral feeding products by launching the Infinity® Orange™ small volume enteral feeding pump. The Infinity Orange is an enteral-only feeding system that has been optimized for use in clinical intensive care units, homecare environments, or anywhere small volume feedings are administered. The Infinity Orange improves patient safety by effectively reducing the possibility of accidental feeding into an IV line or injection of IV medication into a feeding tube. |
| • For more information please click here |
|
• For more information please click here |
| |
| |
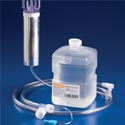 Hudson RCI’s Comfort Flo Humidification System is a fully approved revolutionary device designed to comfortably deliver flows of 1-40LPM of heated, humidified oxygen through a nasal cannula interface. This unique system allows spontaneously breathing patients to comfortably receive the required range of flows to deter from more expensive therapies like CPAP or ventilation. A completely disposable system, equipped with sterile Concha-Column and utilizing sterile Concha water, intended to minimize the fear of cross-contamination and bacteria growth. Hudson RCI’s Comfort Flo Humidification System is a fully approved revolutionary device designed to comfortably deliver flows of 1-40LPM of heated, humidified oxygen through a nasal cannula interface. This unique system allows spontaneously breathing patients to comfortably receive the required range of flows to deter from more expensive therapies like CPAP or ventilation. A completely disposable system, equipped with sterile Concha-Column and utilizing sterile Concha water, intended to minimize the fear of cross-contamination and bacteria growth. |
|
 AorTech International plc, the biomaterials and medical device development company, announced that one of its key customers, Avalon Laboratories, LLC has received 510(k) approval from the US Food and Drug Administration (FDA) for general market release of their specialty Avalon Elite Catheter line of products made exclusively with Elast-Eon. This announcement follows previous releases made by AorTech and Avalon Laboratories and highlights continued developments between the companies. AorTech International plc, the biomaterials and medical device development company, announced that one of its key customers, Avalon Laboratories, LLC has received 510(k) approval from the US Food and Drug Administration (FDA) for general market release of their specialty Avalon Elite Catheter line of products made exclusively with Elast-Eon. This announcement follows previous releases made by AorTech and Avalon Laboratories and highlights continued developments between the companies. |
| • For more information please click here |
|
• For more information please click here |


