| MEDSEEK to Implement Comprehensive Four-Portal eHealth ecoSystem at Rex Healthcare |
|
 |
Birmingham, AL - MEDSEEK, the leading provider of enterprise-wide healthcare portal connectivity solutions, announced that Rex Healthcare signed a multi-year agreement with MEDSEEK to design and implement a comprehensive eHealth ecoSystem, a virtual community in which patients, physicians and employees can collaborate to better manage health and more efficiently deliver health care providing "the right information to the right person at the right time". By making it easier for physicians and patients to interact with the health system, and with each other, the ecosystem solutions will dramatically enhance constituent satisfaction and provide Rex Healthcare with a unique advantage in the rapidly expanding and highly competitive Raleigh, North Carolina area. Committed to recruiting, retaining and affiliating with top-notch physicians, the progressive leaders at Rex Healthcare first turned to MEDSEEK because of the vendor's proven ability to deliver clinical portals that dramatically enhance physician satisfaction. |
| • For more information please click here |
|
| MORE NEWS |
|
 |
| Wright Medical Group, Inc. Receives FDA Clearance for PRO-STIM Osteoinductive Bone Graft Substitute |
|
Aperio Partners with HistoRx to Provide Integrated Fluorescence Whole Slide Imaging System |
 Arlington, TN - Wright Medical Group, Inc., a global orthopaedic medical device company, announced the 510K clearance of PRO-STIM Injectable Osteoinductive Bone Graft Substitute. PRO-STIM graft is a composite grafting material that is injected through a small needle, hardens, and is replaced by the patient's new bone over time. Building on the clinical success of Wright's PRO-DENSE® material platform, PRO-STIM graft provides surgeons with the osteoconductive base material derived from PRO-DENSE® graft (a patent pending combination of calcium sulfate and calcium phosphate materials), but adds a high volume of osteoinductive demineralized bone matrix (DBM) to the formulation. In Wright's pivotal pre-clinical testing, PRO-STIM™ graft outperformed autograft - long considered the grafting "gold standard" - at 13 weeks. "Our pre-clinical model showed accelerated healing compared to autograft, suggesting a superiority to autograft that could be very beneficial for human use in the restoration of skeletal or bone defects," said Thomas Turner, D.V.M., Assistant Professor at Rush University Medical Center in Chicago and principle investigator for the pre-clinical model. Arlington, TN - Wright Medical Group, Inc., a global orthopaedic medical device company, announced the 510K clearance of PRO-STIM Injectable Osteoinductive Bone Graft Substitute. PRO-STIM graft is a composite grafting material that is injected through a small needle, hardens, and is replaced by the patient's new bone over time. Building on the clinical success of Wright's PRO-DENSE® material platform, PRO-STIM graft provides surgeons with the osteoconductive base material derived from PRO-DENSE® graft (a patent pending combination of calcium sulfate and calcium phosphate materials), but adds a high volume of osteoinductive demineralized bone matrix (DBM) to the formulation. In Wright's pivotal pre-clinical testing, PRO-STIM™ graft outperformed autograft - long considered the grafting "gold standard" - at 13 weeks. "Our pre-clinical model showed accelerated healing compared to autograft, suggesting a superiority to autograft that could be very beneficial for human use in the restoration of skeletal or bone defects," said Thomas Turner, D.V.M., Assistant Professor at Rush University Medical Center in Chicago and principle investigator for the pre-clinical model. |
|
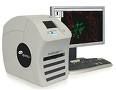 Vista, CA and New Haven, CT - Aperio Technologies, Inc., a global leader in digital pathology for the healthcare and life sciences industry, and HistoRx, Inc., a leader in the emerging field of tissue-based assays for targeted patient treatment, have entered into a partnership to provide an integrated solution for whole-slide imaging with fluorescence. The partnership integrates Aperio's new ScanScope® FL, a multi-slide fluorescence scanning system designed to meet the growing demand for multiplexing with fluorescent biomarkers and quantitative analysis, with HistoRx' AQUA® (Automated Quantitative Analysis) software for fluorescent image analysis that provides in-depth scientific insights from biomarker measurements. Aperio will be a value-added reseller of the AQUA software. Aperio CEO Dirk G. Soenksen said, "With HistoRx, we see an opportunity to meet the growing trend in scientific research and drug discovery toward the use of fluorochrome-labeled tissue markers for diagnosis, treatment selection and monitoring in cancer." Vista, CA and New Haven, CT - Aperio Technologies, Inc., a global leader in digital pathology for the healthcare and life sciences industry, and HistoRx, Inc., a leader in the emerging field of tissue-based assays for targeted patient treatment, have entered into a partnership to provide an integrated solution for whole-slide imaging with fluorescence. The partnership integrates Aperio's new ScanScope® FL, a multi-slide fluorescence scanning system designed to meet the growing demand for multiplexing with fluorescent biomarkers and quantitative analysis, with HistoRx' AQUA® (Automated Quantitative Analysis) software for fluorescent image analysis that provides in-depth scientific insights from biomarker measurements. Aperio will be a value-added reseller of the AQUA software. Aperio CEO Dirk G. Soenksen said, "With HistoRx, we see an opportunity to meet the growing trend in scientific research and drug discovery toward the use of fluorochrome-labeled tissue markers for diagnosis, treatment selection and monitoring in cancer." |
| • For more information please click here |
|
• For more information please click here |
| |
| St. Francis Medical Center Selects Premier Healthcare Alliance Web-based Infection Tracking Solution to Increase Patient Safety, Reduce costs |
|
NuSil Technology Debuts Ultra-Soft Elastomer with Gel-like Properties |
 Charlotte, NC - The Premier healthcare alliance announced that Bon Secours St. Francis Medical Center of Midlothian, Va., selected Premier's SafetySurveillor® Web-based infection tracking and antimicrobial utilization tool. More than 270 hospitals use SafetySurveillor to help prevent healthcare-associated infections (HAIs) and optimize antibiotic use, thereby protecting patients more effectively while safely reducing hospital costs. "We chose Premier for its strong infection prevention expertise and we believe this solution will help facilitate our goal to reduce HAIs," said Mark Gordon executive vice president and administrator of St. Francis Medical Center. "St. Francis has a strong commitment to quality improvement and cost reduction and we are excited to work with Premier." According to Premier's Scott D. Pope, Pharm.D., SafetySurveillor national director, "SafetySurveillor is a technology infection preventionists have relied upon for nearly a decade to increase their efficiency, reduce infection rates and automate surveillance efforts. Charlotte, NC - The Premier healthcare alliance announced that Bon Secours St. Francis Medical Center of Midlothian, Va., selected Premier's SafetySurveillor® Web-based infection tracking and antimicrobial utilization tool. More than 270 hospitals use SafetySurveillor to help prevent healthcare-associated infections (HAIs) and optimize antibiotic use, thereby protecting patients more effectively while safely reducing hospital costs. "We chose Premier for its strong infection prevention expertise and we believe this solution will help facilitate our goal to reduce HAIs," said Mark Gordon executive vice president and administrator of St. Francis Medical Center. "St. Francis has a strong commitment to quality improvement and cost reduction and we are excited to work with Premier." According to Premier's Scott D. Pope, Pharm.D., SafetySurveillor national director, "SafetySurveillor is a technology infection preventionists have relied upon for nearly a decade to increase their efficiency, reduce infection rates and automate surveillance efforts. |
|
 Carpinteria, CA - NuSil Technology LLC, a leading formulator and manufacturer of silicone compounds for the healthcare and pharmaceutical industries, announced the release of MED-4286, an ultra-soft elastomer with a feel similar to a firm gel but greater physical properties, providing a product ideal for potting, encapsulating or molding devices. MED-4286 is a pliable silicone elastomer designed for applications requiring low "000" durometer, as well as low modulus and high elongation. Featuring a low, pourable viscosity and 12-hour work time, MED-4286 is easily moldable, providing versatility for various applications. This material is designated as Unrestricted and can be considered for use in long-term implants (implanted 29 days or longer). "MED-4286 is a notable material for applications that require a soft, yet moldable material that can keep its form," said Brian Nash, vice president of Marketing and Sales. Carpinteria, CA - NuSil Technology LLC, a leading formulator and manufacturer of silicone compounds for the healthcare and pharmaceutical industries, announced the release of MED-4286, an ultra-soft elastomer with a feel similar to a firm gel but greater physical properties, providing a product ideal for potting, encapsulating or molding devices. MED-4286 is a pliable silicone elastomer designed for applications requiring low "000" durometer, as well as low modulus and high elongation. Featuring a low, pourable viscosity and 12-hour work time, MED-4286 is easily moldable, providing versatility for various applications. This material is designated as Unrestricted and can be considered for use in long-term implants (implanted 29 days or longer). "MED-4286 is a notable material for applications that require a soft, yet moldable material that can keep its form," said Brian Nash, vice president of Marketing and Sales. |
| • For more information please click here |
|
• For more information please click here |
| |
| AorTech Signs Licensing Agreement with SynCardia for Elast-Eon Polymer Heart Valve |
|
Baxter Receives EMEA Positive Opinion for CELVAPAN H1N1 Pandemic Influenza Vaccine |
 Surbiton, Surrey, England - AorTech International plc, the biomaterials and medical device development company, announced an exclusive technology licensing and product supply agreement with SynCardia Systems, Inc., manufacturer of the SynCardia temporary CardioWest™ Total Artificial Heart. This agreement licenses the use of AorTech's Elast-Eon™ polymer heart valve (PHV) in the SynCardia Total Artificial Heart, as well as the company's future family of pulsatile products. As part of the larger program of preparing the PHV for both surgical and trans-catheter applications, the PHV has been tested extensively and has satisfied FDA pre-requisites for human use. The AorTech Polymer Heart Valve has been shown to have durability superior to that of biologic valves and comparable hemodynamic characteristics. In these studies, it has consistently demonstrated a remarkable freedom from calcification or thrombus formation. Due to its outstanding operating efficiency, the valve requires less pressure and therefore less power to open and close. Among other benefits, it is anticipated that the valve's efficiency will result in longer times between battery changes and quieter operation, helping improve quality of life for Total Artificial Heart patients while they wait for a matching donor heart. Surbiton, Surrey, England - AorTech International plc, the biomaterials and medical device development company, announced an exclusive technology licensing and product supply agreement with SynCardia Systems, Inc., manufacturer of the SynCardia temporary CardioWest™ Total Artificial Heart. This agreement licenses the use of AorTech's Elast-Eon™ polymer heart valve (PHV) in the SynCardia Total Artificial Heart, as well as the company's future family of pulsatile products. As part of the larger program of preparing the PHV for both surgical and trans-catheter applications, the PHV has been tested extensively and has satisfied FDA pre-requisites for human use. The AorTech Polymer Heart Valve has been shown to have durability superior to that of biologic valves and comparable hemodynamic characteristics. In these studies, it has consistently demonstrated a remarkable freedom from calcification or thrombus formation. Due to its outstanding operating efficiency, the valve requires less pressure and therefore less power to open and close. Among other benefits, it is anticipated that the valve's efficiency will result in longer times between battery changes and quieter operation, helping improve quality of life for Total Artificial Heart patients while they wait for a matching donor heart. |
|
 Deerfield, IL - Baxter International Inc. announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMEA) granted its "positive opinion" for CELVAPAN H1N1 pandemic vaccine using Baxter's Vero cell technology. This positive opinion confirms the acceptability of Baxter's regulatory submission to obtain final marketing authorization and licensure of the product. CELVAPAN H1N1 is the first cell culture-based and non-adjuvanted vaccine to receive a positive opinion in the European Union. Initial quantities of vaccine have already been delivered to a number of countries, including the UK and Ireland, for use in their national vaccination programs, and are awaiting product release subject to final marketing authorization being granted by the European Commission. Presently, Baxter is confirming the safety and immunogenicity of CELVAPAN H1N1 in clinical trials. The company is conducting two randomized trials in 400 healthy adults age 18 and over and in 400 children and adolescents to supplement the licensure post-approval with appropriate clinical data. Deerfield, IL - Baxter International Inc. announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMEA) granted its "positive opinion" for CELVAPAN H1N1 pandemic vaccine using Baxter's Vero cell technology. This positive opinion confirms the acceptability of Baxter's regulatory submission to obtain final marketing authorization and licensure of the product. CELVAPAN H1N1 is the first cell culture-based and non-adjuvanted vaccine to receive a positive opinion in the European Union. Initial quantities of vaccine have already been delivered to a number of countries, including the UK and Ireland, for use in their national vaccination programs, and are awaiting product release subject to final marketing authorization being granted by the European Commission. Presently, Baxter is confirming the safety and immunogenicity of CELVAPAN H1N1 in clinical trials. The company is conducting two randomized trials in 400 healthy adults age 18 and over and in 400 children and adolescents to supplement the licensure post-approval with appropriate clinical data. |
| • For more information please click here |
|
• For more information please click here |
| |
| Initiate Systems Launches Provider Management Solution Helping Healthcare Organizations create Provider Registry and Streamline Data |
|
Zoll Medical Awared U.S. Defense Agency Defibrillator Contract |
 CHICAGO - Initiate Systems, Inc., a leader in data management solutions for the exchange of health information, announced the launch of its Initiate® Provider Management solution, a unique offering that allows healthcare organizations to create a provider registry to ensure that all systems using provider data operate from the same up-to-date information.Like patient data, provider data exists across a healthcare network in multiple disparate systems, such as human resources, credentialing and provisioning, licensing and various laboratory systems. This data often is incompatible between internal systems, causing redundancy and inconsistency. As a result, communication with providers regarding patient care is delayed and information is sometimes misrouted. This issue can cause providers to discontinue patient referrals, causing a negative impact on a health system's bottom line. "Initiate Provider Management is ideal for hospitals with multiple credentialing systems that want to align license, specialization and contact information across their enterprise," said Brian Vile, Initiate's vice president, product strategy. CHICAGO - Initiate Systems, Inc., a leader in data management solutions for the exchange of health information, announced the launch of its Initiate® Provider Management solution, a unique offering that allows healthcare organizations to create a provider registry to ensure that all systems using provider data operate from the same up-to-date information.Like patient data, provider data exists across a healthcare network in multiple disparate systems, such as human resources, credentialing and provisioning, licensing and various laboratory systems. This data often is incompatible between internal systems, causing redundancy and inconsistency. As a result, communication with providers regarding patient care is delayed and information is sometimes misrouted. This issue can cause providers to discontinue patient referrals, causing a negative impact on a health system's bottom line. "Initiate Provider Management is ideal for hospitals with multiple credentialing systems that want to align license, specialization and contact information across their enterprise," said Brian Vile, Initiate's vice president, product strategy. |
|
 Chelmsford, MA - ZOLL Medical Corporation, a manufacturer of resuscitation devices and related software solutions, announced that it has been awarded a follow-on contract for Airworthy CCT defibrillators by the U.S. Defense Logistics Agency. If fully exercised, the potential revenue from the contract could approximate $30 million. The services that can order units against this contract are Army, Navy, Air Force, Marine Corps, and federal civilian agencies. The Department of Defense will provide an upfront payment of $3.9 million for ZOLL to procure parts to build units to comply with inventory requirements. This payment will initially be included in deferred revenue. Recognition of revenue will occur depending upon how and when future orders are placed. ZOLL received a similar award in 2004, with four one-year extension options, all of which were exercised. This new agreement, initially for a one-year period, also contains four one-year agreement extension options. Chelmsford, MA - ZOLL Medical Corporation, a manufacturer of resuscitation devices and related software solutions, announced that it has been awarded a follow-on contract for Airworthy CCT defibrillators by the U.S. Defense Logistics Agency. If fully exercised, the potential revenue from the contract could approximate $30 million. The services that can order units against this contract are Army, Navy, Air Force, Marine Corps, and federal civilian agencies. The Department of Defense will provide an upfront payment of $3.9 million for ZOLL to procure parts to build units to comply with inventory requirements. This payment will initially be included in deferred revenue. Recognition of revenue will occur depending upon how and when future orders are placed. ZOLL received a similar award in 2004, with four one-year extension options, all of which were exercised. This new agreement, initially for a one-year period, also contains four one-year agreement extension options. |
| • For more information please click here |
|
• For more information please click here |
| |
| Roche: Two New Studies Report Increased Survival Rates in Tamiflu Treated Patients with Avian and Severe Seasonal Flu |
|
Physio-Control Receives Market Approval from Health Canada for LIFEPAK 15 Monitor/Defibrillator |
 Basel, Switzerland - Tamiflu (oseltamivir) demonstrated significantly improved survival rates in patients with the highly pathogenic H5N1 influenza (avian flu) and severe cases of seasonal influenza, according to two new studies presented at the 49th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) in San Francisco. "In two different observational studies, the data continues to indicate that there is clinical benefit provided by Tamiflu, including improving survival in patients with H5N1 infection or seasonal influenza," said Jean-Jacques Garaud, Global Head of Pharma Development at Roche. "Both studies reinforce the important role that Tamiflu can provide particularly among the most vulnerable patients and those infected with the most deadly strains”. This is the first multinational study to systematically assess human H5N1 infection. 53% of patients with H5N1 infection survived when treated with Tamiflu compared with 12% of untreated patients. Rate of death was reduced by 37% in high-risk patients with severe seasonal flu compared with no treatment. Basel, Switzerland - Tamiflu (oseltamivir) demonstrated significantly improved survival rates in patients with the highly pathogenic H5N1 influenza (avian flu) and severe cases of seasonal influenza, according to two new studies presented at the 49th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) in San Francisco. "In two different observational studies, the data continues to indicate that there is clinical benefit provided by Tamiflu, including improving survival in patients with H5N1 infection or seasonal influenza," said Jean-Jacques Garaud, Global Head of Pharma Development at Roche. "Both studies reinforce the important role that Tamiflu can provide particularly among the most vulnerable patients and those infected with the most deadly strains”. This is the first multinational study to systematically assess human H5N1 infection. 53% of patients with H5N1 infection survived when treated with Tamiflu compared with 12% of untreated patients. Rate of death was reduced by 37% in high-risk patients with severe seasonal flu compared with no treatment. |
|
 Redmond, WA - Pysio-Control, Inc., a wholly owned subsidiary of Medtronic, Inc., announced it received approval by Health Canada to market the LIFEPAK 15 monitor/defibrillator within Canada. Official license notification was received on Sept. 1, 2009. Physio-Control was granted CE mark in January 2009, certifying compliance with the European Union Medical Device Directive and began its U.S. market release of the LIFEPAK 15 monitor/defibrillator in March 2009. The LIFEPAK 15 monitor/defibrillator builds on a 54-year Physio-Control heritage of providing innovative, reliable equipment to lifesaving teams around the world. With an all-new product platform, it delivers best-in-class clinical and operational innovations and raises the bar on the legendary LIFEPAK® standard for durability. Clinically innovative, the LIFEPAK 15 provides the widest range of energy dosing up to 360 joules, and the broadest selection of monitoring options available. Redmond, WA - Pysio-Control, Inc., a wholly owned subsidiary of Medtronic, Inc., announced it received approval by Health Canada to market the LIFEPAK 15 monitor/defibrillator within Canada. Official license notification was received on Sept. 1, 2009. Physio-Control was granted CE mark in January 2009, certifying compliance with the European Union Medical Device Directive and began its U.S. market release of the LIFEPAK 15 monitor/defibrillator in March 2009. The LIFEPAK 15 monitor/defibrillator builds on a 54-year Physio-Control heritage of providing innovative, reliable equipment to lifesaving teams around the world. With an all-new product platform, it delivers best-in-class clinical and operational innovations and raises the bar on the legendary LIFEPAK® standard for durability. Clinically innovative, the LIFEPAK 15 provides the widest range of energy dosing up to 360 joules, and the broadest selection of monitoring options available. |
| • For more information please click here |
|
• For more information please click here |
| |
| EFD's New Ultimus V High-precision Dispenser & Optimeter Increase Productivity in Critical Assembly Processes |
|
Genzyme's Campath Meets Primary Endpoint in Phase 3 Combination Therapy Trial for Chronic Lymphocytic Leukemia |
 East Providence, RI - EFD, Inc., a subsidiary of Nordson Corporation has developed two new products for improving medical device, electronics and other critical assembly processes. The global medical device market has been estimated at US $200 billion in 2008, while the 2012 global consumer electronics market is forecast to have by 2012 a value of $260.7 billion, an increase of 30.6% since 2007. In today's competitive global environment where advanced products like medical devices and electronics are becoming ever smaller and more complex, manufacturers are challenged with finding new ways to apply the adhesives and other fluids used to build them with greater precision, at faster speeds. Keeping the amount of fluid applied consistent as it gets thicker or thinner, or as the volume of fluid in the reservoir decreases, can be critical to maintaining productivity and quality. EFD's Ultimus V and Optimeter resolve these issues and reduce downtime at automated dispensing stations. East Providence, RI - EFD, Inc., a subsidiary of Nordson Corporation has developed two new products for improving medical device, electronics and other critical assembly processes. The global medical device market has been estimated at US $200 billion in 2008, while the 2012 global consumer electronics market is forecast to have by 2012 a value of $260.7 billion, an increase of 30.6% since 2007. In today's competitive global environment where advanced products like medical devices and electronics are becoming ever smaller and more complex, manufacturers are challenged with finding new ways to apply the adhesives and other fluids used to build them with greater precision, at faster speeds. Keeping the amount of fluid applied consistent as it gets thicker or thinner, or as the volume of fluid in the reservoir decreases, can be critical to maintaining productivity and quality. EFD's Ultimus V and Optimeter resolve these issues and reduce downtime at automated dispensing stations. |
|
 Cambridge, MA - Genzyme Corporation announced that its randomized Phase 3 clinical trial investigating Campath (alemtuzumab) in combination with Fludara (fludarabine phosphate) in relapsed or refractory chronic lymphocytic leukemia (CLL) patients met its primary endpoint by demonstrating a significant improvement in progression free survival (PFS). The trial's independent data safety monitoring board, in a pre-planned interim analysis, determined that the study achieved its primary endpoint and recommended early closure of the trial. Patients in the international, multi-center study treated with Campath in combination with Fludara (FluCAM) experienced a significant increase in the amount of time they lived without the disease progressing compared to patients treated with Fludara alone. The trial was designed to detect at least a 50 percent overall improvement in progression free survival in the FluCAM arm in comparison to the Fludara arm. Study results from this interim analysis are expected to be submitted to the American Society of Hematology meeting held in December. Cambridge, MA - Genzyme Corporation announced that its randomized Phase 3 clinical trial investigating Campath (alemtuzumab) in combination with Fludara (fludarabine phosphate) in relapsed or refractory chronic lymphocytic leukemia (CLL) patients met its primary endpoint by demonstrating a significant improvement in progression free survival (PFS). The trial's independent data safety monitoring board, in a pre-planned interim analysis, determined that the study achieved its primary endpoint and recommended early closure of the trial. Patients in the international, multi-center study treated with Campath in combination with Fludara (FluCAM) experienced a significant increase in the amount of time they lived without the disease progressing compared to patients treated with Fludara alone. The trial was designed to detect at least a 50 percent overall improvement in progression free survival in the FluCAM arm in comparison to the Fludara arm. Study results from this interim analysis are expected to be submitted to the American Society of Hematology meeting held in December. |
| • For more information please click here |
|
• For more information please click here |
| |
| WellSpan Health's York Hospital Drives Enterprise-Wide Operational Efficiency Using AeroScout's Wi-Fi-based Solution |
|
Medical Trial: St. Jude Medical's PressureWire Technology Reduces Heart Attack Risk by 34% When Used Prior to Procedure |
 Redwood City, CA - AeroScout, the leading provider of Unified Asset Visibility for the healthcare industry, announced that WellSpan Health's York Hospital has deployed AeroScout's Wi-Fi-based healthcare visibility solution. York Hospital's emergency, biomedical engineering and transportation departments are using the AeroScout solution to track critical medical equipment throughout the facility to ensure that staff can quickly locate required items. The solution and its time-saving benefits are proving to be especially valuable in the emergency department during peak hours by helping to increase efficiency while improving patient satisfaction, care and safety. York Hospital is a 580-bed, 1.2 million square-foot community teaching hospital that employs more than 3,400 people. It is currently being upgraded to one of only two Level 1 Trauma centers in south-central Pennsylvania. The hospital had wanted to implement a real-time location system (RTLS) for the last five years. Redwood City, CA - AeroScout, the leading provider of Unified Asset Visibility for the healthcare industry, announced that WellSpan Health's York Hospital has deployed AeroScout's Wi-Fi-based healthcare visibility solution. York Hospital's emergency, biomedical engineering and transportation departments are using the AeroScout solution to track critical medical equipment throughout the facility to ensure that staff can quickly locate required items. The solution and its time-saving benefits are proving to be especially valuable in the emergency department during peak hours by helping to increase efficiency while improving patient satisfaction, care and safety. York Hospital is a 580-bed, 1.2 million square-foot community teaching hospital that employs more than 3,400 people. It is currently being upgraded to one of only two Level 1 Trauma centers in south-central Pennsylvania. The hospital had wanted to implement a real-time location system (RTLS) for the last five years. |
|
 St. Paul, MN -- (BUSINESS WIRE) -- St. Jude Medical, Inc. announced that the two-year results from the landmark FAME (Fractional Flow Reserve (FFR) vs. Angiography in Multivessel Evaluation) trial demonstrated the durability of the improved outcomes noted at one-year for patients with multivessel coronary artery disease whose treatment was guided by FFR rather than by standard angiography alone. Two-year results demonstrated that the risk of death or myocardial infarction (heart attack) was 34% lower for patients whose treatment was guided by St. Jude Medical's PressureWire technology prior to coronary stenting. The two-year results demonstrated that patients who received FFR-guided treatment had increasingly superior outcomes over time; the two patient groups had a difference in patient death and myocardial infarction of 3.8% after 12 months and 4.3% after two years. St. Paul, MN -- (BUSINESS WIRE) -- St. Jude Medical, Inc. announced that the two-year results from the landmark FAME (Fractional Flow Reserve (FFR) vs. Angiography in Multivessel Evaluation) trial demonstrated the durability of the improved outcomes noted at one-year for patients with multivessel coronary artery disease whose treatment was guided by FFR rather than by standard angiography alone. Two-year results demonstrated that the risk of death or myocardial infarction (heart attack) was 34% lower for patients whose treatment was guided by St. Jude Medical's PressureWire technology prior to coronary stenting. The two-year results demonstrated that patients who received FFR-guided treatment had increasingly superior outcomes over time; the two patient groups had a difference in patient death and myocardial infarction of 3.8% after 12 months and 4.3% after two years. |
| • For more information please click here |
|
• For more information please click here |
|
| SeqWright: Accelerate Research & Drive Innovation |
|
 |
Founded in 1994 by John W. Belmont, M.D., Ph.D. and Richard Gibbs, Ph.D., Director of the Human Genome Sequencing Center, SeqWright Inc. is a Contract Research Organization (CRO) specializing in Genomic Services. From its inception, Fei Lu, M.D. has run the company, fostering its growth from a small start-up, focused primarily on sequencing, to a powerful full-service Nucleic Acid Technology CRO with services ranging from Molecular Biology, to Microarray, to Next Generation Genomics. SeqWright is CLIA certified and GLP compliant, which enables us to offer FDA and GLP-level services; a strength we have leveraged to provide Clinical Trial support services to the Pharmaceutical Industry and Genomics Services to the USDA. With over 14 years of Genomics experience, we have earned a reputation for quality service, technical expertise, and a willingness to customize services to meet our client's individual needs. |
| • For more information please click here |
|
| OTHER NEWS |
|
 |
|
|
|


 Arlington, TN - Wright Medical Group, Inc., a global orthopaedic medical device company, announced the 510K clearance of PRO-STIM Injectable Osteoinductive Bone Graft Substitute. PRO-STIM graft is a composite grafting material that is injected through a small needle, hardens, and is replaced by the patient's new bone over time. Building on the clinical success of Wright's PRO-DENSE® material platform, PRO-STIM graft provides surgeons with the osteoconductive base material derived from PRO-DENSE® graft (a patent pending combination of calcium sulfate and calcium phosphate materials), but adds a high volume of osteoinductive demineralized bone matrix (DBM) to the formulation. In Wright's pivotal pre-clinical testing, PRO-STIM™ graft outperformed autograft - long considered the grafting "gold standard" - at 13 weeks. "Our pre-clinical model showed accelerated healing compared to autograft, suggesting a superiority to autograft that could be very beneficial for human use in the restoration of skeletal or bone defects," said Thomas Turner, D.V.M., Assistant Professor at Rush University Medical Center in Chicago and principle investigator for the pre-clinical model.
Arlington, TN - Wright Medical Group, Inc., a global orthopaedic medical device company, announced the 510K clearance of PRO-STIM Injectable Osteoinductive Bone Graft Substitute. PRO-STIM graft is a composite grafting material that is injected through a small needle, hardens, and is replaced by the patient's new bone over time. Building on the clinical success of Wright's PRO-DENSE® material platform, PRO-STIM graft provides surgeons with the osteoconductive base material derived from PRO-DENSE® graft (a patent pending combination of calcium sulfate and calcium phosphate materials), but adds a high volume of osteoinductive demineralized bone matrix (DBM) to the formulation. In Wright's pivotal pre-clinical testing, PRO-STIM™ graft outperformed autograft - long considered the grafting "gold standard" - at 13 weeks. "Our pre-clinical model showed accelerated healing compared to autograft, suggesting a superiority to autograft that could be very beneficial for human use in the restoration of skeletal or bone defects," said Thomas Turner, D.V.M., Assistant Professor at Rush University Medical Center in Chicago and principle investigator for the pre-clinical model. Vista, CA and New Haven, CT - Aperio Technologies, Inc., a global leader in digital pathology for the healthcare and life sciences industry, and HistoRx, Inc., a leader in the emerging field of tissue-based assays for targeted patient treatment, have entered into a partnership to provide an integrated solution for whole-slide imaging with fluorescence. The partnership integrates Aperio's new ScanScope® FL, a multi-slide fluorescence scanning system designed to meet the growing demand for multiplexing with fluorescent biomarkers and quantitative analysis, with HistoRx' AQUA® (Automated Quantitative Analysis) software for fluorescent image analysis that provides in-depth scientific insights from biomarker measurements. Aperio will be a value-added reseller of the AQUA software. Aperio CEO Dirk G. Soenksen said, "With HistoRx, we see an opportunity to meet the growing trend in scientific research and drug discovery toward the use of fluorochrome-labeled tissue markers for diagnosis, treatment selection and monitoring in cancer."
Vista, CA and New Haven, CT - Aperio Technologies, Inc., a global leader in digital pathology for the healthcare and life sciences industry, and HistoRx, Inc., a leader in the emerging field of tissue-based assays for targeted patient treatment, have entered into a partnership to provide an integrated solution for whole-slide imaging with fluorescence. The partnership integrates Aperio's new ScanScope® FL, a multi-slide fluorescence scanning system designed to meet the growing demand for multiplexing with fluorescent biomarkers and quantitative analysis, with HistoRx' AQUA® (Automated Quantitative Analysis) software for fluorescent image analysis that provides in-depth scientific insights from biomarker measurements. Aperio will be a value-added reseller of the AQUA software. Aperio CEO Dirk G. Soenksen said, "With HistoRx, we see an opportunity to meet the growing trend in scientific research and drug discovery toward the use of fluorochrome-labeled tissue markers for diagnosis, treatment selection and monitoring in cancer." Surbiton, Surrey, England - AorTech International plc, the biomaterials and medical device development company, announced an exclusive technology licensing and product supply agreement with SynCardia Systems, Inc., manufacturer of the SynCardia temporary CardioWest™ Total Artificial Heart. This agreement licenses the use of AorTech's Elast-Eon™ polymer heart valve (PHV) in the SynCardia Total Artificial Heart, as well as the company's future family of pulsatile products. As part of the larger program of preparing the PHV for both surgical and trans-catheter applications, the PHV has been tested extensively and has satisfied FDA pre-requisites for human use. The AorTech Polymer Heart Valve has been shown to have durability superior to that of biologic valves and comparable hemodynamic characteristics. In these studies, it has consistently demonstrated a remarkable freedom from calcification or thrombus formation. Due to its outstanding operating efficiency, the valve requires less pressure and therefore less power to open and close. Among other benefits, it is anticipated that the valve's efficiency will result in longer times between battery changes and quieter operation, helping improve quality of life for Total Artificial Heart patients while they wait for a matching donor heart.
Surbiton, Surrey, England - AorTech International plc, the biomaterials and medical device development company, announced an exclusive technology licensing and product supply agreement with SynCardia Systems, Inc., manufacturer of the SynCardia temporary CardioWest™ Total Artificial Heart. This agreement licenses the use of AorTech's Elast-Eon™ polymer heart valve (PHV) in the SynCardia Total Artificial Heart, as well as the company's future family of pulsatile products. As part of the larger program of preparing the PHV for both surgical and trans-catheter applications, the PHV has been tested extensively and has satisfied FDA pre-requisites for human use. The AorTech Polymer Heart Valve has been shown to have durability superior to that of biologic valves and comparable hemodynamic characteristics. In these studies, it has consistently demonstrated a remarkable freedom from calcification or thrombus formation. Due to its outstanding operating efficiency, the valve requires less pressure and therefore less power to open and close. Among other benefits, it is anticipated that the valve's efficiency will result in longer times between battery changes and quieter operation, helping improve quality of life for Total Artificial Heart patients while they wait for a matching donor heart. Deerfield, IL - Baxter International Inc. announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMEA) granted its "positive opinion" for CELVAPAN H1N1 pandemic vaccine using Baxter's Vero cell technology. This positive opinion confirms the acceptability of Baxter's regulatory submission to obtain final marketing authorization and licensure of the product. CELVAPAN H1N1 is the first cell culture-based and non-adjuvanted vaccine to receive a positive opinion in the European Union. Initial quantities of vaccine have already been delivered to a number of countries, including the UK and Ireland, for use in their national vaccination programs, and are awaiting product release subject to final marketing authorization being granted by the European Commission. Presently, Baxter is confirming the safety and immunogenicity of CELVAPAN H1N1 in clinical trials. The company is conducting two randomized trials in 400 healthy adults age 18 and over and in 400 children and adolescents to supplement the licensure post-approval with appropriate clinical data.
Deerfield, IL - Baxter International Inc. announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMEA) granted its "positive opinion" for CELVAPAN H1N1 pandemic vaccine using Baxter's Vero cell technology. This positive opinion confirms the acceptability of Baxter's regulatory submission to obtain final marketing authorization and licensure of the product. CELVAPAN H1N1 is the first cell culture-based and non-adjuvanted vaccine to receive a positive opinion in the European Union. Initial quantities of vaccine have already been delivered to a number of countries, including the UK and Ireland, for use in their national vaccination programs, and are awaiting product release subject to final marketing authorization being granted by the European Commission. Presently, Baxter is confirming the safety and immunogenicity of CELVAPAN H1N1 in clinical trials. The company is conducting two randomized trials in 400 healthy adults age 18 and over and in 400 children and adolescents to supplement the licensure post-approval with appropriate clinical data. Chelmsford, MA - ZOLL Medical Corporation, a manufacturer of resuscitation devices and related software solutions, announced that it has been awarded a follow-on contract for Airworthy CCT defibrillators by the U.S. Defense Logistics Agency. If fully exercised, the potential revenue from the contract could approximate $30 million. The services that can order units against this contract are Army, Navy, Air Force, Marine Corps, and federal civilian agencies. The Department of Defense will provide an upfront payment of $3.9 million for ZOLL to procure parts to build units to comply with inventory requirements. This payment will initially be included in deferred revenue. Recognition of revenue will occur depending upon how and when future orders are placed. ZOLL received a similar award in 2004, with four one-year extension options, all of which were exercised. This new agreement, initially for a one-year period, also contains four one-year agreement extension options.
Chelmsford, MA - ZOLL Medical Corporation, a manufacturer of resuscitation devices and related software solutions, announced that it has been awarded a follow-on contract for Airworthy CCT defibrillators by the U.S. Defense Logistics Agency. If fully exercised, the potential revenue from the contract could approximate $30 million. The services that can order units against this contract are Army, Navy, Air Force, Marine Corps, and federal civilian agencies. The Department of Defense will provide an upfront payment of $3.9 million for ZOLL to procure parts to build units to comply with inventory requirements. This payment will initially be included in deferred revenue. Recognition of revenue will occur depending upon how and when future orders are placed. ZOLL received a similar award in 2004, with four one-year extension options, all of which were exercised. This new agreement, initially for a one-year period, also contains four one-year agreement extension options. Basel, Switzerland - Tamiflu (oseltamivir) demonstrated significantly improved survival rates in patients with the highly pathogenic H5N1 influenza (avian flu) and severe cases of seasonal influenza, according to two new studies presented at the 49th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) in San Francisco. "In two different observational studies, the data continues to indicate that there is clinical benefit provided by Tamiflu, including improving survival in patients with H5N1 infection or seasonal influenza," said Jean-Jacques Garaud, Global Head of Pharma Development at Roche. "Both studies reinforce the important role that Tamiflu can provide particularly among the most vulnerable patients and those infected with the most deadly strains”. This is the first multinational study to systematically assess human H5N1 infection. 53% of patients with H5N1 infection survived when treated with Tamiflu compared with 12% of untreated patients. Rate of death was reduced by 37% in high-risk patients with severe seasonal flu compared with no treatment.
Basel, Switzerland - Tamiflu (oseltamivir) demonstrated significantly improved survival rates in patients with the highly pathogenic H5N1 influenza (avian flu) and severe cases of seasonal influenza, according to two new studies presented at the 49th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) in San Francisco. "In two different observational studies, the data continues to indicate that there is clinical benefit provided by Tamiflu, including improving survival in patients with H5N1 infection or seasonal influenza," said Jean-Jacques Garaud, Global Head of Pharma Development at Roche. "Both studies reinforce the important role that Tamiflu can provide particularly among the most vulnerable patients and those infected with the most deadly strains”. This is the first multinational study to systematically assess human H5N1 infection. 53% of patients with H5N1 infection survived when treated with Tamiflu compared with 12% of untreated patients. Rate of death was reduced by 37% in high-risk patients with severe seasonal flu compared with no treatment. Redmond, WA - Pysio-Control, Inc., a wholly owned subsidiary of Medtronic, Inc., announced it received approval by Health Canada to market the LIFEPAK 15 monitor/defibrillator within Canada. Official license notification was received on Sept. 1, 2009. Physio-Control was granted CE mark in January 2009, certifying compliance with the European Union Medical Device Directive and began its U.S. market release of the LIFEPAK 15 monitor/defibrillator in March 2009. The LIFEPAK 15 monitor/defibrillator builds on a 54-year Physio-Control heritage of providing innovative, reliable equipment to lifesaving teams around the world. With an all-new product platform, it delivers best-in-class clinical and operational innovations and raises the bar on the legendary LIFEPAK® standard for durability. Clinically innovative, the LIFEPAK 15 provides the widest range of energy dosing up to 360 joules, and the broadest selection of monitoring options available.
Redmond, WA - Pysio-Control, Inc., a wholly owned subsidiary of Medtronic, Inc., announced it received approval by Health Canada to market the LIFEPAK 15 monitor/defibrillator within Canada. Official license notification was received on Sept. 1, 2009. Physio-Control was granted CE mark in January 2009, certifying compliance with the European Union Medical Device Directive and began its U.S. market release of the LIFEPAK 15 monitor/defibrillator in March 2009. The LIFEPAK 15 monitor/defibrillator builds on a 54-year Physio-Control heritage of providing innovative, reliable equipment to lifesaving teams around the world. With an all-new product platform, it delivers best-in-class clinical and operational innovations and raises the bar on the legendary LIFEPAK® standard for durability. Clinically innovative, the LIFEPAK 15 provides the widest range of energy dosing up to 360 joules, and the broadest selection of monitoring options available. East Providence, RI - EFD, Inc., a subsidiary of Nordson Corporation has developed two new products for improving medical device, electronics and other critical assembly processes. The global medical device market has been estimated at US $200 billion in 2008, while the 2012 global consumer electronics market is forecast to have by 2012 a value of $260.7 billion, an increase of 30.6% since 2007. In today's competitive global environment where advanced products like medical devices and electronics are becoming ever smaller and more complex, manufacturers are challenged with finding new ways to apply the adhesives and other fluids used to build them with greater precision, at faster speeds. Keeping the amount of fluid applied consistent as it gets thicker or thinner, or as the volume of fluid in the reservoir decreases, can be critical to maintaining productivity and quality. EFD's Ultimus V and Optimeter resolve these issues and reduce downtime at automated dispensing stations.
East Providence, RI - EFD, Inc., a subsidiary of Nordson Corporation has developed two new products for improving medical device, electronics and other critical assembly processes. The global medical device market has been estimated at US $200 billion in 2008, while the 2012 global consumer electronics market is forecast to have by 2012 a value of $260.7 billion, an increase of 30.6% since 2007. In today's competitive global environment where advanced products like medical devices and electronics are becoming ever smaller and more complex, manufacturers are challenged with finding new ways to apply the adhesives and other fluids used to build them with greater precision, at faster speeds. Keeping the amount of fluid applied consistent as it gets thicker or thinner, or as the volume of fluid in the reservoir decreases, can be critical to maintaining productivity and quality. EFD's Ultimus V and Optimeter resolve these issues and reduce downtime at automated dispensing stations. Cambridge, MA - Genzyme Corporation announced that its randomized Phase 3 clinical trial investigating Campath (alemtuzumab) in combination with Fludara (fludarabine phosphate) in relapsed or refractory chronic lymphocytic leukemia (CLL) patients met its primary endpoint by demonstrating a significant improvement in progression free survival (PFS). The trial's independent data safety monitoring board, in a pre-planned interim analysis, determined that the study achieved its primary endpoint and recommended early closure of the trial. Patients in the international, multi-center study treated with Campath in combination with Fludara (FluCAM) experienced a significant increase in the amount of time they lived without the disease progressing compared to patients treated with Fludara alone. The trial was designed to detect at least a 50 percent overall improvement in progression free survival in the FluCAM arm in comparison to the Fludara arm. Study results from this interim analysis are expected to be submitted to the American Society of Hematology meeting held in December.
Cambridge, MA - Genzyme Corporation announced that its randomized Phase 3 clinical trial investigating Campath (alemtuzumab) in combination with Fludara (fludarabine phosphate) in relapsed or refractory chronic lymphocytic leukemia (CLL) patients met its primary endpoint by demonstrating a significant improvement in progression free survival (PFS). The trial's independent data safety monitoring board, in a pre-planned interim analysis, determined that the study achieved its primary endpoint and recommended early closure of the trial. Patients in the international, multi-center study treated with Campath in combination with Fludara (FluCAM) experienced a significant increase in the amount of time they lived without the disease progressing compared to patients treated with Fludara alone. The trial was designed to detect at least a 50 percent overall improvement in progression free survival in the FluCAM arm in comparison to the Fludara arm. Study results from this interim analysis are expected to be submitted to the American Society of Hematology meeting held in December. St. Paul, MN -- (BUSINESS WIRE) -- St. Jude Medical, Inc. announced that the two-year results from the landmark FAME (Fractional Flow Reserve (FFR) vs. Angiography in Multivessel Evaluation) trial demonstrated the durability of the improved outcomes noted at one-year for patients with multivessel coronary artery disease whose treatment was guided by FFR rather than by standard angiography alone. Two-year results demonstrated that the risk of death or myocardial infarction (heart attack) was 34% lower for patients whose treatment was guided by St. Jude Medical's PressureWire technology prior to coronary stenting. The two-year results demonstrated that patients who received FFR-guided treatment had increasingly superior outcomes over time; the two patient groups had a difference in patient death and myocardial infarction of 3.8% after 12 months and 4.3% after two years.
St. Paul, MN -- (BUSINESS WIRE) -- St. Jude Medical, Inc. announced that the two-year results from the landmark FAME (Fractional Flow Reserve (FFR) vs. Angiography in Multivessel Evaluation) trial demonstrated the durability of the improved outcomes noted at one-year for patients with multivessel coronary artery disease whose treatment was guided by FFR rather than by standard angiography alone. Two-year results demonstrated that the risk of death or myocardial infarction (heart attack) was 34% lower for patients whose treatment was guided by St. Jude Medical's PressureWire technology prior to coronary stenting. The two-year results demonstrated that patients who received FFR-guided treatment had increasingly superior outcomes over time; the two patient groups had a difference in patient death and myocardial infarction of 3.8% after 12 months and 4.3% after two years.